
ADA 2025 Recap: Key Themes from the Scientific Sessions
In this ADA 2025 recap, Orange Biomed exchanged key insights with industry experts while presenting our latest innovation at the 85th Scientific Sessions of the American Diabetes Association (ADA), held June 20–23, 2025, in Chicago. As one of the most anticipated global events in diabetes innovation and research, this year’s conference gathered leaders from across the industry to explore the latest advances in technology, care models, and patient advocacy.
At the heart of Orange Biomed’s (OBM) presence was our featured session at the Innovation Hub, titled “Solving HbA1c Monitoring Challenges: Bringing Lab-Level Accuracy to the Home.” We demonstrated how our novel solution, OBM rapid A1c, leverages microfluidic technology to offer lab-quality A1C testing at home, making this innovative tool more accessible for millions, particularly those with limited access to primary care.
In this article, we’re recapping key highlights from our experience at ADA 2025.
ADA 2025 Recap - Day 1:
We kicked off the conference with a live interview, recorded directly from the Innovation Hub on the exhibit floor to be broadcast in the upcoming weeks by Stacey Simms, who hosts the Diabetes Connections podcast. The insights shared by Orange Biomed with Simms will be broadcast on the podcast in the upcoming weeks.
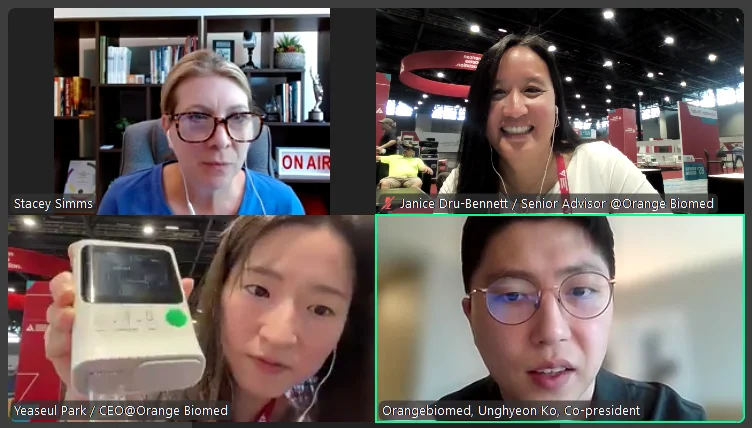
Stacey Simms meets with Orange Biomed on the Exhibit Floor during the opening day of the American Diabetes Association (ADA) 85th Scientific Sessions on June 20, 2025, to see firsthand the OBM rapid A1c device showcased at the Innovation Hub.
Following the kickoff interview, our team held a series of impactful meetings across both ADA and nearby partner venues with fellow diabetes advocates. In our meeting with Terra A. Campbell, the Associate Director at Howard Brown Health, we discussed the latest innovations in diabetes technology and how solutions can reach more populations in clinics across Chicago.

The Orange Biomed Team Visited Howard Brown Health
It was valuable to discuss these important topics with Terra and we’re excited to continue sharing insights and providing resources with her and other partners at the M.A.P. Your Health Chicago: Community Forum & Public Health Resource Fair in August.
We also explored topics around influencing diabetes policy and empowering advocates, as well as the value of having an at-home A1C testing device to reach rural and underserved communities, with members from the Diabetes Patient Advocacy Coalition, including Erin M. Callahan (COO), George Huntley (CEO), Nancy Hondt, Larry Smith, R. Stewart Perry, and Bruce Taylor.
Day 2:
We started Saturday and Sunday bright and early with the ADA Group 5K Run at Museum Campus, connecting with fellow attendees and healthcare professionals with Chuck Henderson, Chief Executive Officer of the American Diabetes Association.

The ADA ADA Group 5K Run
Photo Source: Tracs Inc
Photo Source: Tracs Inc
In the afternoon, Orange Biomed Co-President Dr. Unghyeon Ko took the stage for our Innovation Hub Presentation, “Solving HbA1c Monitoring Challenges: Bringing Lab-Level Accuracy to the Home.” Ko outlined the technical and systemic barriers people with diabetes face in getting regular A1C tests and how OBM’s solution is designed to overcome them.

Dr. Unghyeon Ko Presented a Breakthrough A1C Testing Method at the Innovation Hub
Dr. Ko highlighted that “Numerous clinical studies show that consistently monitoring A1C is critical for reducing the risk of diabetes-related complications. However, many people fail to undergo regular A1C testing. Accessibility to diagnostics remains a significant barrier for 47% of the global population, and over 34 million Americans living in primary care deserts. Yet the inherent limitations of conventional biochemical A1C measurement methods have hindered efforts to improve accessibility without sacrificing accuracy.”
“To fundamentally resolve the accessibility issue of A1C testing, a completely new measurement approach is required. Orange Biomed is addressing this challenge by developing an innovative A1C technology—a breakthrough microfluidics-based device, in addition to launching a national movement to mobilize communities to develop proactive care approaches,” added Dr. Ko.
Throughout the day, our team continued to engage with a range of stakeholders across the diabetes ecosystem.
It was a pleasure to connect with Stephanie Kim, an Endocrinologist and Professor at the University of Washington, Sindhu Rajan, PhD, from HabitNu, and representatives from Novo Nordisk, Boehringer Ingelheim, GLUCO, and other organizations to showcase how our innovative A1C testing method can improve accessibility and community health outcomes.
We additionally enjoyed the opportunity to discuss one of the biggest challenges in the U.S. healthcare system with Eric Ogg from Abbott. During our conversation, we addressed a key structural barrier in the U.S.: insurance policies that require patients to travel to external labs for A1C testing, causing delays, drop-offs, and missed diagnoses. OBM’s at-home model offers a clear path to closing that gap.
Day 3:
Our last day at the ADA Scientific Sessions was filled with engaging conversations focused on advancing diabetes screening and testing. In our conversations with Ny’Nika McFadden, PhD, Schafer Boeder, and Obinna Ekwunife, we discussed how OBM rapid A1c will transform the future of diabetes management and opportunities to collaborate in the future.
We had a great experience at ADA and are very grateful for the opportunity to present at the Innovation Hub. The conference additionally allowed us the opportunity to discuss important topics with diabetes professionals and advocates. Orange Biomed is excited to continue building on the conversations we had about improving the accessibility of diabetes testing and screening, especially for marginalized communities.
Join us in the discussion by checking out our M.A.P. Your Health campaign and sharing your health journey story.







